Advancing Safer, Low-Cost Batteries for Grid Energy Storage
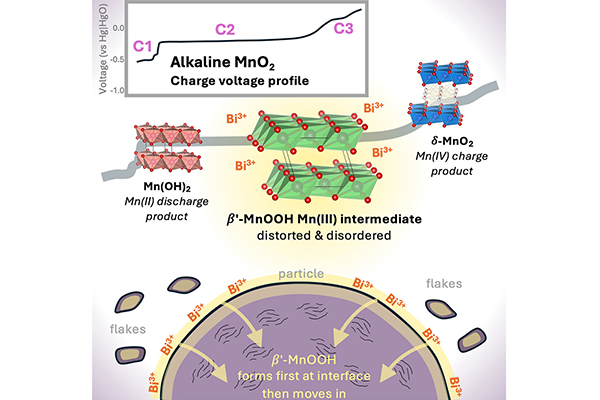
Chemical engineering student Eric Zimmerer, PhD’26, co-authored a paper with Rachana Somaskandan, E’25, Connor Fawcett, PhD’29, Assistant Professor Qing Zhao, and Associate Professor Joshua Gallaway on the “Dynamics of disordered intermediates during the two-electron alkaline MnO₂ conversion reaction for grid-scale batteries,” which was published in Joule. Their research uncovers how manganese dioxide (MnO₂) cathodes store and release energy in rechargeable alkaline batteries, identifying the disordered Mn(III) intermediates that form during cycling and providing key insights to engineer safer, lower-cost batteries for large-scale energy storage.
Abstract:
Battery technologies beyond Li-ion are likely needed for extensive integration of grid-scale storage. The rechargeable Zn-MnO2 chemistry has the potential for high sustainability, high safety, and low cost, using Earth-abundant basis materials. In an alkaline electrolyte, the MnO2 cathode can cycle reversibly if modified by including a Bi-containing additive, although the cycling mechanism remains mostly unknown. This work presents an account of the intermediate species involved in the electrochemical transformation from layered δ-MnO2 to Mn(OH)2 and back. During charge, a disordered intermediate with a structure resembling layered β-MnOOH exists stably for an extended period, corresponding to a regime known to have unexpected electrochemical activity of Bi. During discharge, β-MnOOH exists only briefly and is never the majority material, revealing that the cycling mechanism is asymmetric. These findings represent a significant advance in mechanistic knowledge and can enable engineering to develop the system for commercial use.